Navigating the Chemical Landscape: A Stoichiometry Roadmap
Related Articles: Navigating the Chemical Landscape: A Stoichiometry Roadmap
Introduction
With enthusiasm, let’s navigate through the intriguing topic related to Navigating the Chemical Landscape: A Stoichiometry Roadmap. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Navigating the Chemical Landscape: A Stoichiometry Roadmap

Stoichiometry, a fundamental concept in chemistry, provides the framework for understanding and predicting the quantitative relationships between reactants and products in chemical reactions. This roadmap, built upon the principles of conservation of mass and definite proportions, allows chemists to precisely determine the amounts of substances involved in chemical transformations.
The Foundation: Understanding the Language of Chemistry
Before embarking on the stoichiometry journey, it’s crucial to grasp the basic vocabulary of chemistry. This includes:
- Chemical Formulae: These symbolic representations depict the elements and their respective ratios within a compound. For instance, H₂O signifies that water comprises two hydrogen atoms and one oxygen atom.
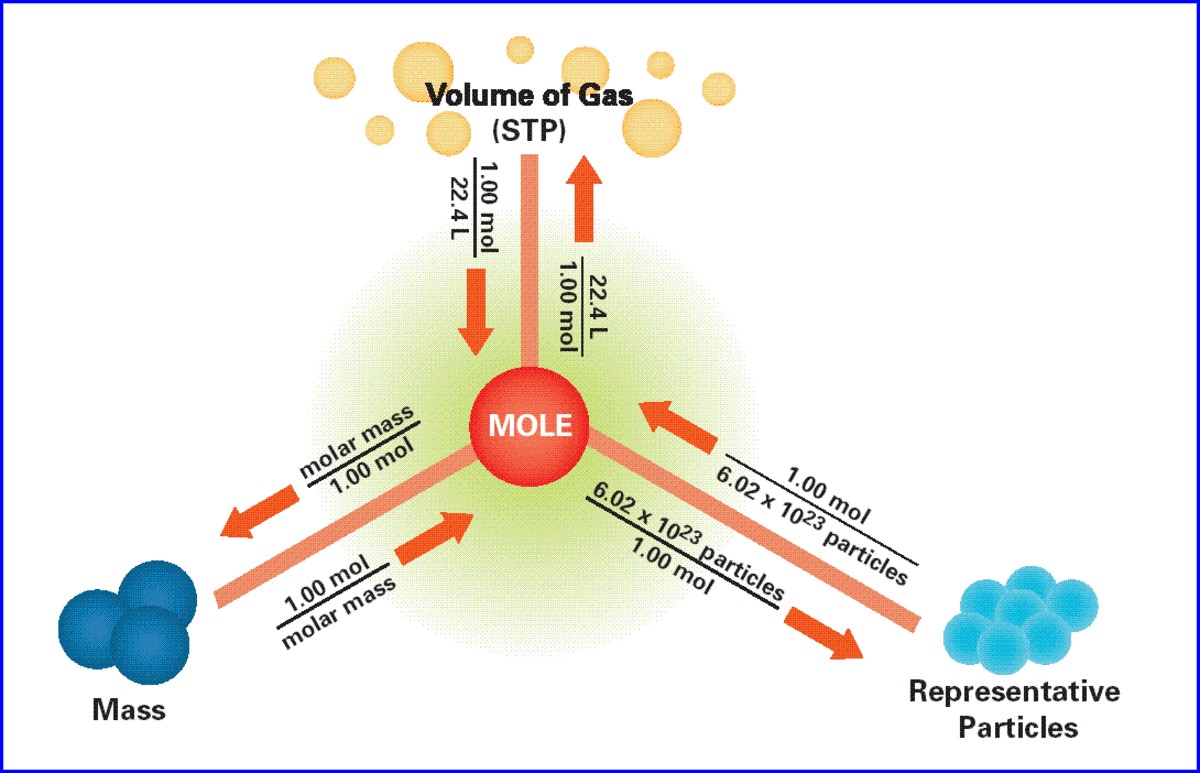
- Moles: A mole represents a specific number of particles (6.022 x 10²³), providing a standardized unit for measuring the amount of substance.
- Molar Mass: The molar mass of a compound is the mass of one mole of that substance. This value is determined by summing the atomic masses of all the atoms in the compound’s formula.
The Stoichiometry Roadmap: A Step-by-Step Guide
The stoichiometry roadmap guides us through the process of analyzing and predicting chemical reactions. It involves the following key steps:
1. Balancing Chemical Equations:
The first step in the stoichiometry roadmap is balancing chemical equations. This ensures that the number of atoms of each element on the reactant side (left side) equals the number of atoms of the same element on the product side (right side). This principle reflects the law of conservation of mass, stating that matter cannot be created or destroyed in chemical reactions.
Example:
The unbalanced equation for the reaction between hydrogen and oxygen to form water is:
H₂ + O₂ → H₂O
To balance this equation, we need to adjust the coefficients in front of each molecule:
2H₂ + O₂ → 2H₂O
Now, there are four hydrogen atoms and two oxygen atoms on both sides of the equation, ensuring mass conservation.
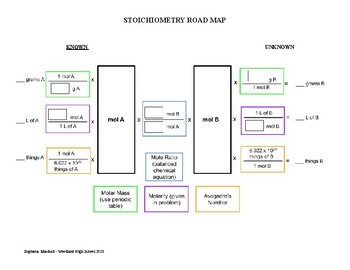
2. Mole Ratios:
Once the equation is balanced, we can extract the mole ratios between the reactants and products. These ratios represent the proportional relationships between the amounts of different substances involved in the reaction.
Example:
In the balanced equation above, the mole ratio between hydrogen (H₂) and water (H₂O) is 2:2, which can be simplified to 1:1. This means that for every one mole of hydrogen consumed, one mole of water is produced.
3. Mass Calculations:
Using molar masses and mole ratios, we can calculate the masses of reactants and products involved in a reaction.
Example:
To calculate the mass of water produced from 4 grams of hydrogen, we follow these steps:
- Convert grams of hydrogen to moles: 4 g H₂ / 2 g/mol H₂ = 2 mol H₂
- Use the mole ratio to find moles of water: 2 mol H₂ x (1 mol H₂O / 1 mol H₂) = 2 mol H₂O
- Convert moles of water to grams: 2 mol H₂O x 18 g/mol H₂O = 36 g H₂O
Therefore, 36 grams of water are produced from 4 grams of hydrogen.
4. Limiting Reactants:
In many reactions, one reactant may be completely consumed before the other. This reactant is called the limiting reactant, as it limits the amount of product that can be formed.
Example:
Consider a reaction where 2 moles of hydrogen react with 1 mole of oxygen. Based on the balanced equation, we need 2 moles of hydrogen for every 1 mole of oxygen. In this case, oxygen is the limiting reactant because it will be completely consumed before all the hydrogen is used.
5. Percent Yield:
The theoretical yield represents the maximum amount of product that can be produced based on stoichiometric calculations. However, in real-world reactions, the actual yield may be less due to factors like incomplete reactions or side reactions. The percent yield reflects the efficiency of the reaction and is calculated as:
Percent Yield = (Actual Yield / Theoretical Yield) x 100%
The Importance of Stoichiometry: A Cornerstone of Chemical Understanding
Stoichiometry plays a pivotal role in various aspects of chemistry, from understanding basic chemical reactions to designing complex industrial processes. Its significance lies in:
- Predicting Reaction Outcomes: Stoichiometry allows us to predict the amount of product that can be formed from a given amount of reactants, crucial for optimizing chemical processes.
- Understanding Reaction Mechanisms: By analyzing the stoichiometry of a reaction, we gain insights into the steps involved in the reaction mechanism, aiding in the development of new synthetic pathways.
- Designing Chemical Experiments: Stoichiometry is essential for planning and executing experiments accurately, ensuring precise measurements and reliable results.
- Industrial Applications: Stoichiometry is fundamental in industries like pharmaceuticals, manufacturing, and agriculture, where optimizing chemical reactions is crucial for efficient production.
FAQs on Stoichiometry
Q1: What is the difference between a chemical formula and a chemical equation?
A1: A chemical formula represents the composition of a specific compound, while a chemical equation describes a chemical reaction, showing the reactants and products involved.
Q2: How do I determine the limiting reactant in a reaction?
A2: Compare the mole ratios of reactants to their actual amounts. The reactant that runs out first is the limiting reactant.
Q3: What are the common errors in stoichiometry calculations?
A3: Common errors include:
- Incorrectly balancing chemical equations.
- Using incorrect mole ratios.
- Neglecting to consider the limiting reactant.
- Misinterpreting units and conversions.
Q4: How can I improve my understanding of stoichiometry?
A4: Practice solving various stoichiometry problems, focusing on understanding the concepts and applying them to real-world scenarios.
Tips for Mastering Stoichiometry
- Thoroughly Understand the Concepts: Ensure a firm grasp of basic chemical concepts like atomic mass, molar mass, and mole ratios before tackling stoichiometry problems.
- Practice Regularly: Consistent practice is key to mastering stoichiometry. Work through numerous problems of varying complexity to solidify your understanding.
- Visualize the Reactions: Draw diagrams or visualize the reactions to better understand the relationships between reactants and products.
- Seek Help When Needed: Don’t hesitate to ask your teacher or a tutor for assistance if you encounter difficulties.
Conclusion
Stoichiometry is a powerful tool in the chemist’s arsenal, providing a roadmap for navigating the complex world of chemical reactions. By understanding the fundamental principles of conservation of mass and definite proportions, we can accurately predict and control the outcomes of chemical transformations. As we delve deeper into the realm of chemistry, stoichiometry remains an essential foundation for unlocking the secrets of the molecular world.





.jpg)
Closure
Thus, we hope this article has provided valuable insights into Navigating the Chemical Landscape: A Stoichiometry Roadmap. We hope you find this article informative and beneficial. See you in our next article!